People are not yeast: Redefining disability in research writing
Language matters. Science is painstaking bench work, but once it's out in the world, how it's communicated shapes how we understand it – and who might be harmed by it.
This study, recently out in PLOS Biology from a yeast genetics lab at the University of Wisconsin, investigated how chromosome duplication affects aging. Escalante et al. found that yeast cells with extra chromosomes have defects entering quiescence (a dormant state that protects cells from stress), leading to premature aging.
It's an elegant study, detailed, persuasively written. And yet an aspect of how it's framed is eating at me. Let's take a look at the abstract.
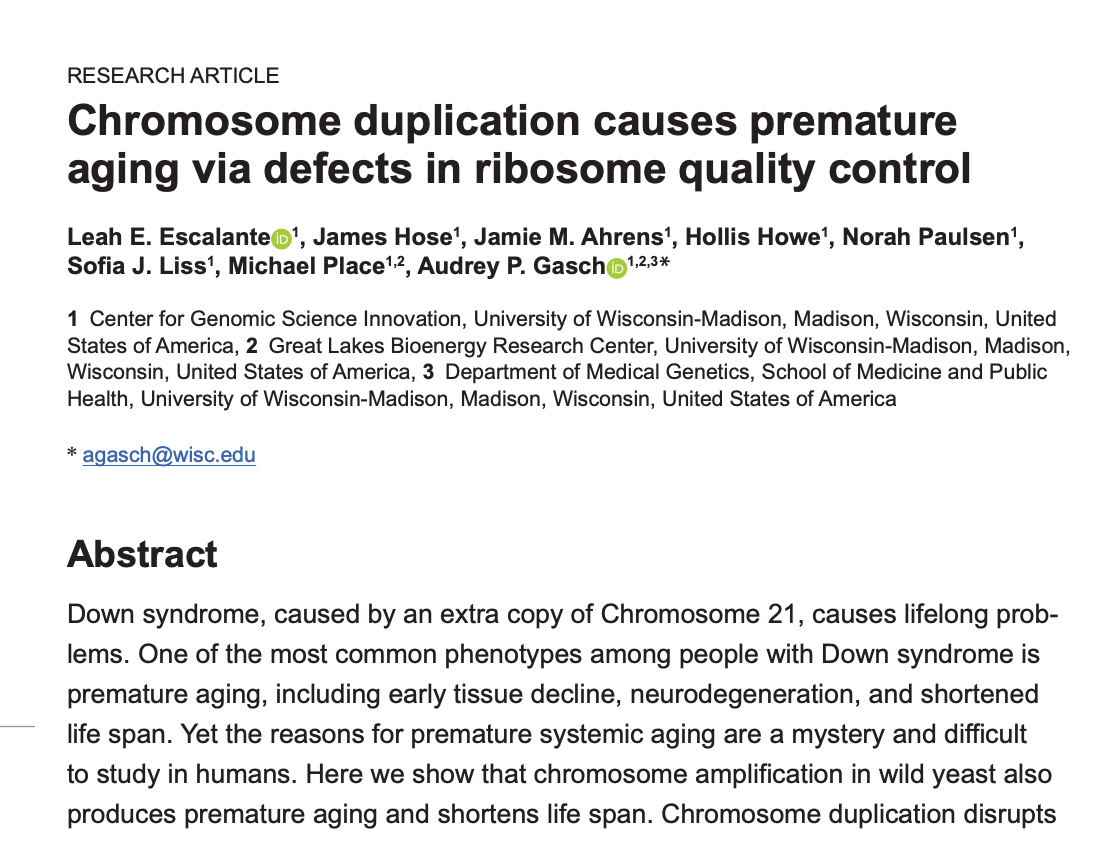
Title and abstract of "Chromosome duplication causes premature aging via defects in ribosome quality control" by Leah E. Escalante et al., PLOS Biology, January 2025. (DOI)
The study opens not with yeast but with Down syndrome:
“Down syndrome, caused by an extra copy of Chromosome 21, causes lifelong problems. One of the most common phenotypes among people with Down syndrome is premature aging, including early tissue decline, neurodegeneration, and shortened life span. Yet the reasons for premature systemic aging are a mystery and difficult to study in humans.”
Ds is being presented as the justification for the study: in article-writing terms, as the rationale. A solid rationale statement will clearly connect the research being reported on (yeast, ribosome quality control) to why it matters (potential implications for people with Ds).
And Escalante et al.'s rationale is solid. It makes logical sense: understanding the effects of aneuploidy (abnormal chromosome number) on aging in yeast can help us understand similar phenomena in people with Ds. And it shows how their work fills an important knowledge gap: "The reasons for premature systemic aging are largely a mystery," they write, "in part because the underlying cellular consequences of chromosome amplification remain unknown despite over 65 years of study."
In other words, this study presents findings that can help understand previously unrecognized mechanisms of premature aging in Ds. That's fantastic. Really well done.
The trouble is that other parts of the article undermine that very rationale and in fact risk alienating the population it means to benefit. To be clear, I don't see evidence of conscious bias and believe that the work is genuinely meant to help the Ds community. Nevertheless, language is particularly tricky when discussing a condition associated with intellectual disability, and this paper (unintentionally) reinforces social biases against Ds.
There are a few subtle patterns here worth noting. We'll walk through them one by one, while looking at alternatives that might have been more successful.
First, regarding the nature of Down syndrome itself. In beginning with the idea that Ds "causes lifelong problems," the paper defines it as intrinsically problematic. There is similar language elsewhere; for example, the introduction notes that chromosome amplification "is very detrimental during mammalian development."
These phrasings I'm drawing attention to subtly embody the deficit model of disability, in which "disability is a medical deficit from a perceived normal." The deficit model would define Ds as problematic by nature and requiring mitigation by medical treatment. At its worst, the deficit model can reinforce biases against people with disabilities, creating barriers to equitable medical care and meaningful social inclusion.
Escalante et al.'s opening nods to problems during development and across the lifespan, but the main focus is on the later years. The authors explain that Ds is commonly associated with "premature aging, including early tissue decline, neurodegeneration, and shortened life span." All true, and their study is aimed at addressing these effects. The problem is that premature aging has already been presented as the culmination of a life marked as problematic, both during development and across the lifespan.
Premature aging, in fact, is presented as the defining characteristic of Ds. I see it here, in the discussion: "Shortened life span is a hallmark of DS." And here, in the introduction: "one of the most penetrant hallmarks of DS ... is premature aging."
A hallmark: a defining feature.
It's not that they're wrong, exactly. But a defining feature? I'd say not. Those of us lucky enough to love someone with Ds might point to the sparkle in their eyes (Brushfield spots), the palmar crease across their hands, their curving pinky fingers. And yes: congenital heart defects, increased rates of childhood cancer. Speech and motor delays, hypotonia, intellectual disability. Also, unavoidably: social stigma, educational inequity, medical stigma, health inequities. Ds does impact people throughout their lives. But shortened lifespan isn't the defining feature.
It is inaccurate and potentially harmful to say that Ds is problematic by nature, no matter the biological facts. Doing so can only exacerbate the inequities that people with Ds already face. Do they live with challenges that others don't? Yes, resoundingly. But defining those challenges as "problematic" can create the misperception that Ds (and people with it) are themselves problematic.
It's important for researchers whose work touches on Ds to affirm the intrinsic worth of individuals in this population. Yes, Ds causes real phenomena that present real challenges and medical implications. But at the same time, it is possible to convey these ideas while handling the disability itself as neutral. For example, from a neuropsychological study of patients with Ds:
"Down syndrome (DS) is the set of phenotypes of variable expressivity that typically results from trisomy 21. People with DS are especially vulnerable to neurodevelopmental and neurodegenerative disorders."
This is more neutral, positioning people with Ds as people first, and it defines Ds neutrally: as a set of phenotypes, which are variable, and which impact but do not characterize people with Ds (the lead author, incidentally, has a child with Ds).
Ds-affirming language like this would have helped Escalante et al. to present their work more neutrally and to position themselves as supporting, rather than trying to fix, people with Ds. Another thing that would have helped – a more transparent connection between what the study found and what it might mean for this population.
Notably, the article does not mention any benefits to people with Ds that might emerge from their findings. Research like this could open up paths to additional research, funding, maybe someday pharmaceutical interventions. But none of that is mentioned. In other words, it defines Ds and premature aging as problems but doesn't position the study as part of the solution.
It would have been an easy loop to close: establish Ds and aging as the study rationale, and circle back later to mention potential positive outcomes for this population. That could be done as a framing device at the start of the discussion, or at the tail end alongside the mention of future research studies. Even if it's speculative, is there any way in which this work might have therapeutic implications? There must be, or else the premise of the study (that yeast is a useful model for human aneuploidy) collapses. The paper is so close. It just needs to take that final step. But instead, it recruits people with Ds as the reason for the study without allowing them to be stakeholders in what it finds.
Historical detour: Louis Pasteur explaining yeast budding in Studies on fermentation: The diseases of beer: “From the ends of a compound organism like those in No. III, a little spherical cell may detach itself and then, by a process of budding, give rise to a series of other rairiute spherical cells reproducing the form shown in Nos. I. and II.”
But if people with Ds aren't the stakeholders, who are? The pessimist in me says: people who study yeast. The study design uses yeast as a model system for chromosome amplification in Ds. I'm not saying that model systems aren't useful, or that they aren't appropriate for studying complex phenomena like the ones Escalante et al. are working with. But the way this article positions yeast relative to humans, in light of the factors discussed above, creates the unwanted impression that, well, yeast are more convenient.
Here's what I mean.
The authors lay out the risks of premature aging in Ds and then rapidly pivot to yeast. Here, from the abstract:
"One of the most common phenotypes among people with Down syndrome is premature aging, including early tissue decline, neurodegeneration, and shortened life span. Here we show that chromosome amplification in wild yeast also produces premature aging and shortens life span."
And in shortened form, from the discussion: "Shortened life span is a hallmark of DS and has also been observed in aneuploidy-sensitized laboratory yeast and yeast with higher ploidy."
Model organisms are tools for understanding humans, not the other way around. But in both cases, Escalante et al.'s syntax reverses that relationship, positioning people first and yeast second. This creates the unintended impression that people with Ds are useful to yeast biologists – a gateway to the yeast research.
In addition, the word "also" in each example creates a functional equivalence between humans and yeast. But the two halves are not equivalent. On one side of the equation, people with Ds – defined as having lifelong problems including neurodegeneration and tissue decline. On the other side of the equation, yeast cells – which age prematurely under chromosome amplification but don't have lifelong problems or tissue decline. The study has already defined Ds as a collection of problems that no amount of yeast research can fix.
By way of contrast, check out the model organism framing in this 2026 study, also published in a PLOS journal. It used a mouse model to study the neurological effects of Zika virus on infants:
"In this study, we adapted a model of maternal ZIKV infection in human STAT2 knock-in (hSTAT2) mice to investigate the effects of ZIKV infection during mid-gestation, aiming to mirror typical asymptomatic infections as they occur in humans."
This phrasing puts the model system first, which clarifies that mice are being used to "mirror" human infections. The utility of the model system is apparent. That doesn't happen in the yeast study. And because Escalante et al. don't position the research as useful for people with Ds, that population becomes defined by their utility to science.
This aspect of the paper would also have been helped with a close-the-loop move connecting the study to its potential payoffs for the Ds population. Doing so would have not only made the work more palatable for people in the Ds community – it would have strengthened its logical foundations.
To step back for a moment: this research is intricate and engaging, and it has real implications for the health of people with Ds. But the way it's framed risks obscuring that message of progress, potentially alienating the people it aims to help while reinforcing disability stigma that already exists within medical research.
So, what to do about research like this? How can researchers whose work has implications for people with disabilities communicate their findings with sensitivity – while not selling their work short?
Good news: it's very possible. Here are some tools.
Cite recent literature. Escalante et al. rely mainly on older literature to characterize premature aging in Ds. For instance, two of the papers cited in the opening paragraph to define the phenomenon are from 1978 and 1985, and they were conducted on institutionalized patients (some of whom were deceased). These references can be cut entirely; the more recent literature cited is more accurate and ethically sound.
Look to models. Major Ds research centers (like this one and this one) exist worldwide and publish widely. Their work, like the neuropsychological study mentioned above, can be used to model sensitive and affirming language.
Use person-first language. Most academic style guides, including the American Medical Association and the American Psychological Association manuals, provide specific guidance on reducing bias and crafting inclusive language.
Invest in a sensitivity read. Hire an editor skilled in language surrounding disability to review your work before you submit it. It's not a big expense, maybe an hour or two of editorial time, and with tangible benefits for how your work will be received.
Escalante et al.: thanks for the work you are doing. As someone writing from within the Ds community, I see the value and the promise of this research. Studies like this have the power to reshape what Ds means, how people understand it, and how people with Ds live their lives. And with language that is careful and affirming, they can do more than drive science forward: they can build trust and partnership with the communities they set out to serve.
I offer sensitivity reads for scientific manuscripts. Contact me to learn more.